

Automated chemical kinetic mechanism simplification with minimal user expertise
R.M. Galassi, P.P. Ciottoli, S.M. Sarathy, H.G. Im, S. Paolucci, M. Valorani
Combustion and Flame 197, pp. 439-448, (2018)
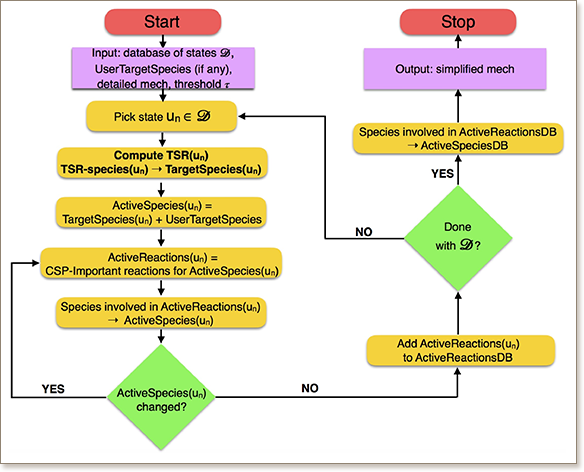
An improved algorithm to generate skeletal mechanisms from the original detailed chemical kinetic mechanisms is proposed. The new algorithm builds on the computational singular perturbation (CSP) framework, by adding an additional layer of automation based on the tangential stretching rate (TSR) and the species’ participation index to TSR. The main advantage of the new approach is that it does not require the specification of a set of target species. Instead, the target species set is dynamic and automatically identified through the simplification algorithm, which defines the system’s state variables that the skeletal mechanism is required to accurately predict. In this way, the new procedure pursues an optimum set of target species that leads the algorithm to include a minimal number of species/reactions that ensures the replication of global observables, such as ignition delay time, without any a-priori knowledge of the chemical pathways. The capabilities and performance of the new simplification algorithm are demonstrated in a test problem employing the 397-species detailed mechanism for C0-C2species, augmented with polycyclic aromatic hydrocarbon (PAH) soot precursor species. The results are compared with those obtained from the standard CSP-based algorithm in terms of the ignition delay times, main species evolution and their equilibrium state. Subsequent examples also demonstrate the capabilities of the TSR-based algorithm to generate additional sub-mechanisms that, combined with the core mechanism, allow to extend its range of operating conditions and target variables.